Multiple Products for Different Needs
Dedicated for best quality innovations
Sterile Infusion Sets for Single Use
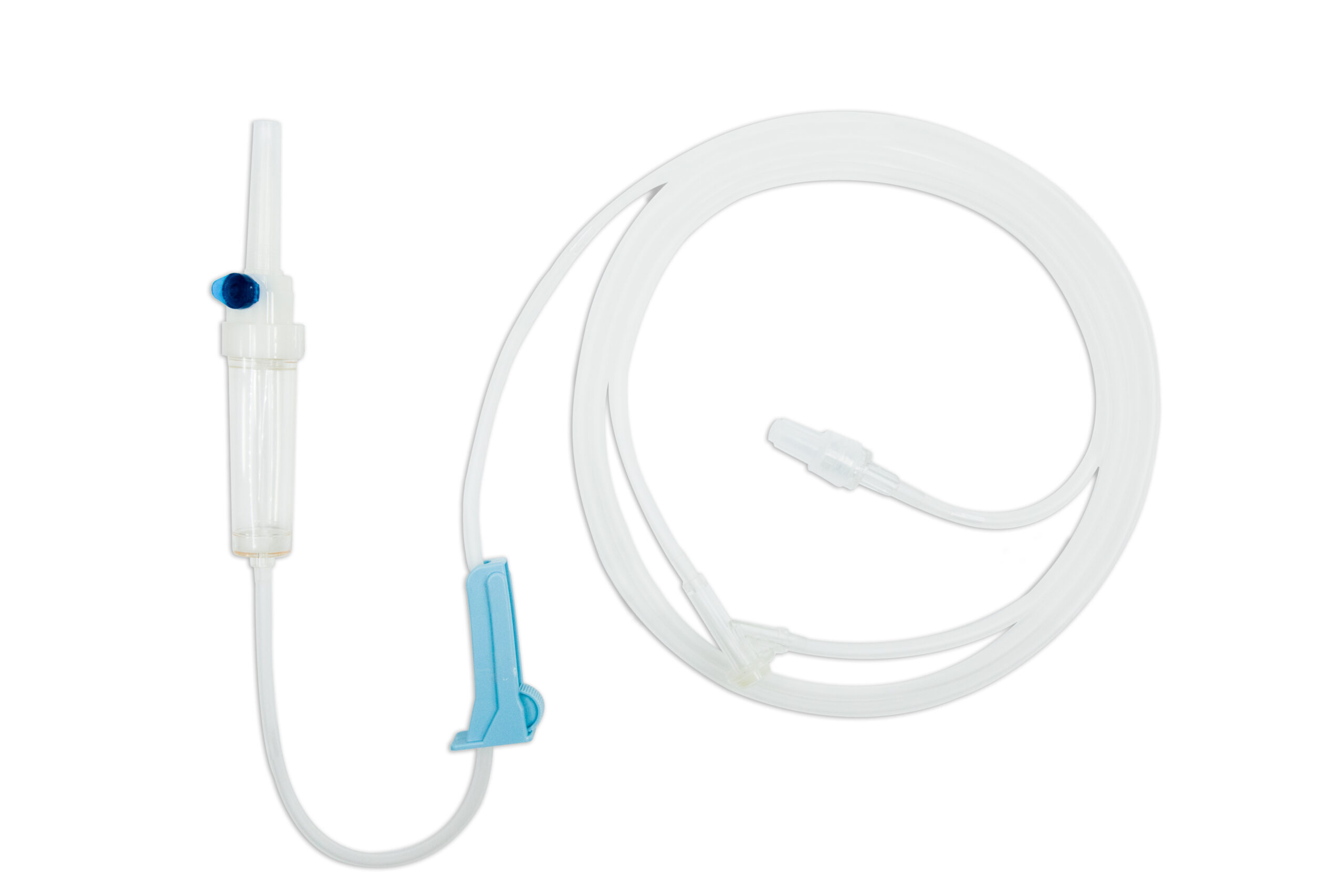
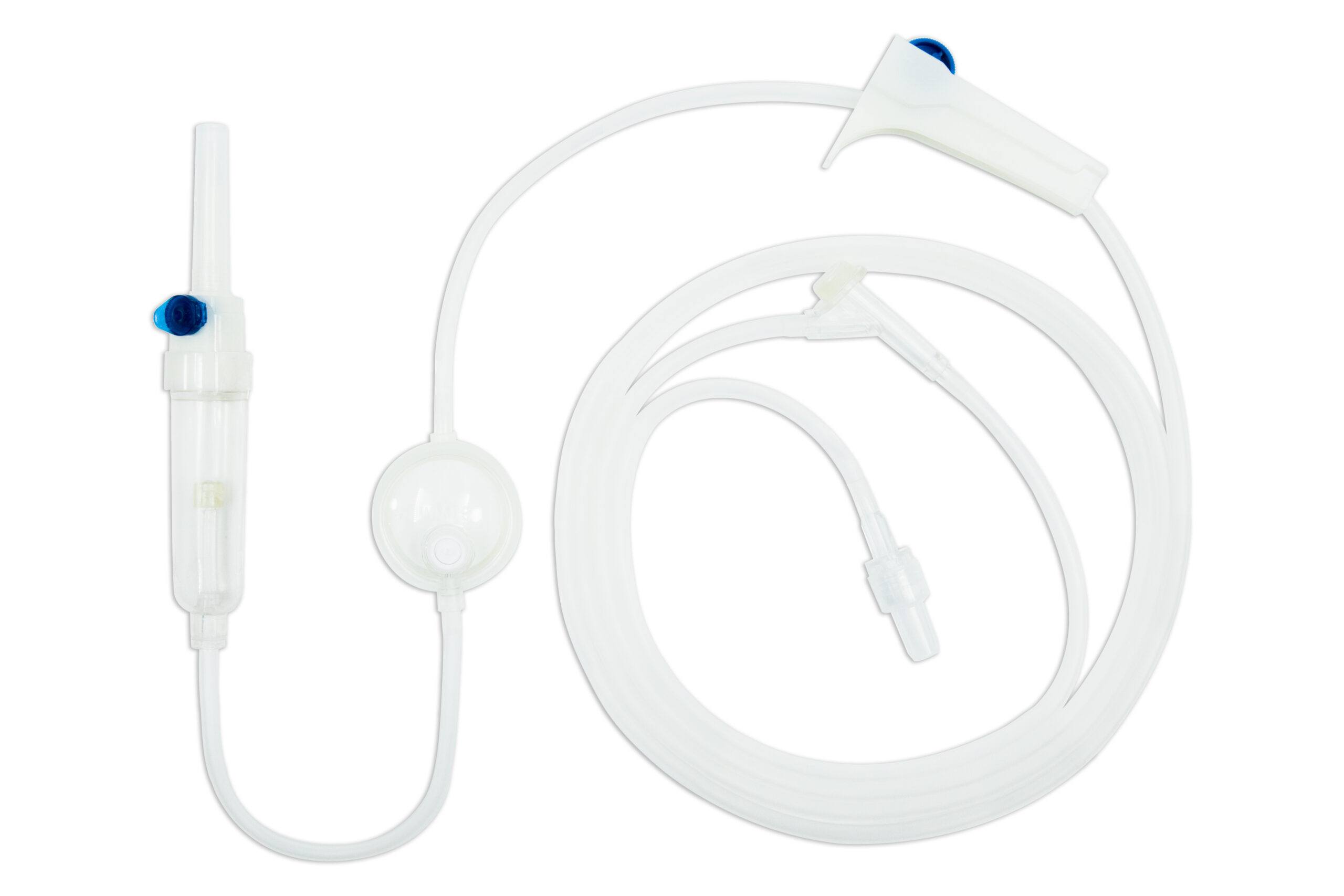
Suyun Medical Infusion Set 100010 is a high-quality, gravity IV infusion system designed for reliable and efficient fluid delivery. This fluid giving set includes a spike, drip chamber, 15μm filter, PVC tubing, Y-injection site, Luer lock adapter, and flow regulator, ensuring precise and safe infusions. Infusion sets with luer locks allow for a more secure link between the sterile infusion set and other devices, reducing the risk of leakage. Its DEHP-free option and customizable tubing lengths (1500–2500mm) cater to diverse healthcare needs. Featuring user-friendly Y-injection for medication delivery during infusion and EO gas sterilization for maximum safety, it’s a versatile solution. Customizable OEM packaging with your branding adds value.
As an experienced medical disposable products manufacturer, Suyun Medical is an expert in disposable injection sets and IV giving sets. In addition to this IV infusion set with a flow regulator, we also manufacture infusion sets with filters, with 3-way stopcocks and so on. To find medical consumables and disposables, contact us today for pricing and to elevate your medical care standards.
Product Details
Type: Standard type with Y-adapter + Luer lock adapter
Product structure: Spike/Drip chamber/15um Filter/Tube/Flow regulator/Y injection site/Adaptor
Application: It is used for gravity infusion
Product feature: The PVC tube is could be with or without DEHP, length could be adjustable according to customer requirement. The Y injection site is used for injection during infusion.
Packing information: we offer customer OEM packing with your logo and company information. Standard packing way is peelable pouch individually,25pcs/big bag,20 big bags/carton.
Sterilization: By E.O. gas
Specification:
Code: | Details |
100010 | Tube length: 1500 ~2500mm Filter: 15.0μm |
Let's start talking with us
Our value
SUYUN Certifications
CE0123
since 2002
ISO13485
since 2002
FDA510K
since 2009
SFDA
since 1995